Introduction Concizumab is a high-affinity, anti-tissue factor pathway inhibitor (TFPI) monoclonal antibody currently in clinical investigation for the once-daily subcutaneous prophylactic treatment of patients with hemophilia. We present results from the combined main and extension parts (at least 76 weeks) of the phase 2 explorer4 trial (NCT03196284), which aimed to assess the safety and longer-term efficacy of concizumab in patients with hemophilia A with inhibitors (HAwI) and hemophilia B with inhibitors (HBwI).
Methods explorer4 comprised a main part, which lasted at least 24 weeks for all patients, and an extension part, which lasted at least 52 weeks. The primary objective of the main part of explorer4 was to assess the efficacy of concizumab in preventing bleeds in patients with HAwI/HBwI, evaluated as annualized bleeding rate (ABR) at the last dose level after at least 24 weeks. This objective has been addressed in previous reporting of the main part of the trial (Shapiro A, et al. Blood 2019; 134[22]:1973-1982). During the main part of the trial, patients were randomized 2:1 to receive either concizumab prophylaxis or on-demand treatment with recombinant activated factor VII (rFVIIa). At the end of the main part, patients in the rFVIIa on-demand arm were switched to 0.15 mg/kg concizumab for the extension part. Concizumab dose was escalated to 0.20 and 0.25 mg/kg in both the main and extension parts if a patient experienced ≥3 treated spontaneous bleeding episodes within 12 weeks and if deemed safe by the investigator. The objective of the extension part of the trial was to assess safety and longer-term efficacy of concizumab. Endpoints included ABR after at least 76 weeks of treatment, concizumab and free TFPI concentrations prior to last dose administration at 76 weeks, number of adverse events (AEs) and occurrence of anti-drug antibodies (ADAs) during 76 weeks of treatment. ABR was estimated using LS-means estimates, based on a negative binomial regression with log of exposure time to concizumab in main and extension part as offset.
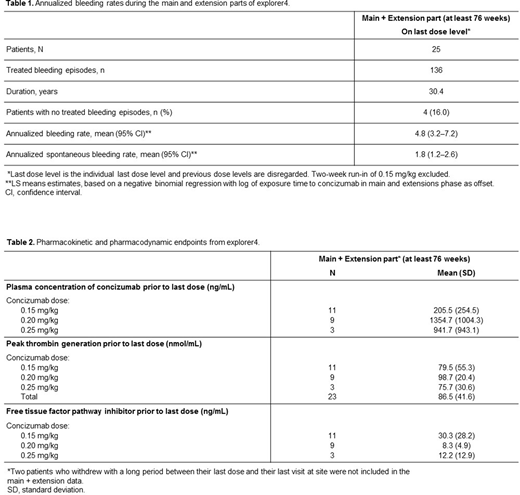
Results Twenty-five patients with inhibitors were exposed to concizumab during the explorer4 trial; 15 with HAwI and 10 with HBwI. Eight of these patients received on-demand treatment with rFVIIa during the main part of the trial before receiving their first concizumab dose in the extension phase. The estimated ABR at the last dose level for all patients treated with concizumab during the main and extension parts was 4.8 (95% CI: 3.2-7.2), while during the trial this was 5.7 (95% CI: 4.2−7.8) (Table 1). During the main and extension parts, 4 (16%) patients had zero treated bleeding episodes on their last dose level. Plasma concentrations of concizumab were variable during the extension part of the trial (Table 2). Increased concizumab concentration was observed in patients who received concizumab 0.20 mg/kg; these patients also had lower concentrations of free TFPI (Table 2). There were no AEs leading to withdrawal, no thromboembolic events and no deaths during the main and extension parts of the trial. ADAs developed in 6 patients. Elevated prothrombin fragment 1+2 and D-dimer levels were observed in some patients treated with concizumab.
Conclusions Results from the combined main and extension parts of the phase 2 explorer4 trial were in line with the clinical proof of concept established in the main part of the trial and provided further details of the safety and longer-term efficacy of subcutaneous prophylactic treatment with concizumab for at least 76 weeks in patients with HAwI and HBwI.
Shapiro:BioMarin: Research Funding; Agios: Research Funding; Genentech/Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Catalyst BioSciences: Membership on an entity's Board of Directors or advisory committees; Sangamo: Research Funding; OPKO: Research Funding; Daiichi Sankyo: Research Funding; Sigilon: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kedrion Biopharma: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees; Bioverativ: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Glover Blood Therapeutics: Research Funding; Octapharma: Research Funding; Pfizer: Research Funding; ProMetic Bio Therapeutics: Consultancy, Research Funding. Castaman:Uniqure: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Ablynx: Honoraria; Alexion: Honoraria; Bayer: Honoraria; Baxalta/Shire: Honoraria; Sobi: Honoraria, Research Funding, Speakers Bureau; Novo Nordisk: Honoraria, Speakers Bureau; Werfen: Speakers Bureau; Kedrion: Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Cepo:Novo Nordisk: Current Employment. Marie Tønder:Novo Nordisk: Current Employment. Matsushita:Sysmex: Honoraria; Octapharm: Honoraria; Bayer: Consultancy, Honoraria; Novo Nordisk: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Shire/Takeda: Honoraria; Bioverative/Sanofi: Honoraria; CSL: Honoraria; JB: Honoraria; KMB: Honoraria; Kirin: Honoraria; Nichiyaku: Honoraria. Young:Bayer, CSL Behring, Freeline, UniQure: Consultancy; BioMarin, Freeline, Genentech/Roche, Grifols, Kedrion, Novo Nordisk, Sanofi Genzyme, Spark, Takeda, and UniQure: Honoraria; Genentech/Roche, Grifols, and Takeda: Research Funding. Zupancic-Šalek:NovoNordisk Health Care AG: Honoraria, Speakers Bureau; Baxter: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Octapharma: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau; Sobi: Honoraria, Speakers Bureau; Bayer: Honoraria, Speakers Bureau. Jimenez Yuste:NovoNordisk: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Grifols: Honoraria, Research Funding; Bayer: Honoraria; Sobi: Consultancy, Honoraria, Research Funding; CSL: Honoraria; Octapharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal